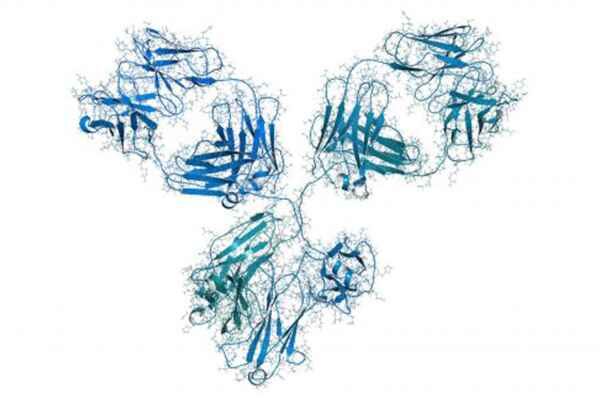
The Argument for Recombinant Antibody Production
Proteos is an industry leader in the production of high-quality recombinant antibodies for drug discovery research. Our previous blog posted in January, discussed some of the limitations of monoclonal antibody (mAb) production in hybridoma cell lines. Here we will focus on the benefits of producing mAbs recombinantly in mammalian cells.
To circumvent some of the shortcomings of hybridomas, recombinant methods for mAb production have been established. Once a mAb amino acid sequence is determined, the coding sequences can be engineered for recombinant production in mammalian cells. The secreted mAb is easily purified from the cell culture supernatant by affinity chromatography to ligands such as Protein A.
There are many advantages to producing mAbs recombinantly in mammalian cells. First, recombinant production ensures a perpetual source of the mAb. Since recombinant expression is contingent upon the mAb sequence, the mAb source is no longer dependent on the viability of the hybridoma. Second, recombinant technology offers the option to make low-cost molecular modifications to the mAb. This includes altering the mAb species through the constant regions or optimizing mAb efficacy by point mutations. In this manner, the recombinant mAb sequence can easily be customized for the experimental application. Furthermore, recombinant mAb yields are more consistent between batches, since expression is driven by the genetic sequence, rather than the immunization of the host animal. Moreover, recombinant production offers higher mAb yields than hybridoma preparations in a shorter period of time. Finally, recombinant technology is ideal for the welfare and ethical treatment of laboratory animals. Once the mAb sequence is determined, there is no longer a need for animal immunizations. For all of these reasons, the recombinant approach to mAb production is increasingly being recognized as an efficient and reliable alternative to hybridomas.
The Proteos team knows that the right building blocks for drug discovery research provide a solid foundation for success. Our antibody production capabilities include transient expression and purification of mAbs. Together, we can explore which of our validated methodologies will best suit your project needs and design a customized workflow to facilitate the highest likelihood of success.
Partner with us to advance your drug discovery research program.